Application Hardening for Medical Devices
Protect the mobile apps that connect to implantables and wearables. Strengthen patient safety, prevent unauthorized control, and meet evolving security expectations with resilient, built-in application security.


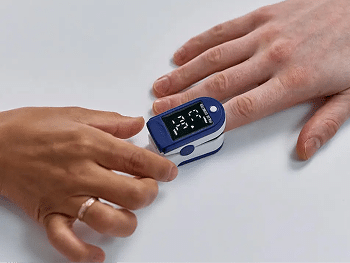
Rising Risks for Connected Medical Devices
Mobile Apps Expose New Attack Paths
Mobile companion apps expand the implantable’s attack surface, enabling unauthorized access if left unprotected.
Tampering Drives Fraud at Scale
Unsecured apps allow determined users to bypass safeguards and issue unsafe or unintended commands.
Safety and Compliance Are at Stake
Tampering or unauthorized control can jeopardize patient safety, cause patient harm, and complicate regulatory obligations.
Purpose-built Security for Medical Apps
Deep Protection Against Unauthorized Control
Digital.ai embeds resilient defenses directly into compiled mobile apps, preventing patients or attackers from issuing unsafe or unintended commands to connected devices. Our injected protections secure the full command path, reducing risks that threaten patient safety.

Tamper Resistance Built Into the App
We make medical companion apps extremely difficult to modify, clone, or sideload. Multiple layers of obfuscation and tamper detection ensure that any attempt to alter app logic or bypass safety constraints is detected and disrupted at runtime.

Resilience Against Modern Reverse Engineering
Dynamic instrumentation, emulators, and analysis toolkits are increasingly used to probe medical apps. Digital.ai’s proprietary code-injection–based protection identifies and blocks these techniques, safeguarding device logic, pairing flows, and communication pathways from exposure.

FIPS-Validated Secure Cryptography and Data Handling
Our platform includes FIPS 140-3–validated White Box Cryptography and secure key handling to protect sensitive logic and device communication. These controls reduce the risk of unauthorized pairing, spoofed telemetry, or manipulation of safety-critical data.

Compliance Support Through Stronger Controls
Digital.ai supports medical device manufacturers with controls aligned to cybersecurity expectations and ISO 13485–compliant processes. Our protection stack helps teams demonstrate due diligence and maintain device safety throughout the software lifecycle.

Learn how our ISO 13485–aligned, FIPS-validated AppSec can strengthen your device ecosystem. Schedule a conversation.
Key Protections for Medical Apps
Block Unauthorized Commands
Prevents unsafe or unintended device actions triggered through compromised or tampered apps.
Stop Reverse Engineering Attempts
Disrupts static and dynamic analysis to protect device logic and communication flows.
Ensure App Integrity
Detects and blocks cloned, modified, or sideloaded versions before harm can occur.
Protect Sensitive Cryptography
FIPS-validated controls safeguard keys, pairing logic, and safety-critical data handling.
Maintain Regulatory Compliance
Supports ISO 13485–aligned processes and evolving FDA cybersecurity expectations.
Rising Attacks on Client Apps—Are Your Defenses Keeping Up?

ISO 13485 Certified
ISO 13485 is an international standard that outlines the requirements for a quality management system (QMS) specifically for the medical device industry. It ensures organizations consistently produce safe and effective medical devices by covering the entire product lifecycle, from design and development to production, installation, and servicing. This standard helps companies meet both customer and applicable regulatory requirements, making it a critical framework for market access.
FIPS 140-3 Validated
Our cryptographic controls meet the U.S. government’s highest verification standard. This validation ensures our protections use proven, rigorously tested cryptography—critical for customers requiring strong data protection, regulated compliance, and confidence in the integrity of their security stack.
FIPS 140-3 is technically harmonized with ISO/IEC 19790, which defines international security requirements for cryptographic modules. In other words, FIPS 140-3 is the U.S./Canada adoption of the same core standard that ISO/IEC 19790 specifies globally.

Related Application Security Resources
Ready to Get Started?
We can make your medical device app resilient to attacks using OWASP MASVS recommended protections.


